Glitter and Neons in Cosmetics – Unsafe Ingredients?
Quite some time ago, I stumbled across this article on TKB Trading’s site, regarding glitter and cosmetics.
The page states:
The FDA has determined that glitter is a color additive which is not listed on their list of approved color additives. This means that a glitter product is not allowed for use in any cosmetic in the USA. Consumers have expressed confusion over this, as it is obvious that there is glitter in all kinds of cosmetics sold currently in the USA and there are no known reports of harm caused by glitter.
The FDA has not explained itself to our company, but it has advised us that it recognizes that the cosmetics industry has been largely unaware of this determination and it is essentially providing the cosmetic industry a grace period during which FDA enforcement is “discretionary”. This grace period allows the cosmetics industry to “respond”. The FDA has not provided us with any information on how long this grace period has been in effect, nor how long it will be in effect. They simply state the issue is “active”.
 Pictured are some of my bright, safe cosmetics.
Pictured are some of my bright, safe cosmetics.
I was shocked by this information (regarding glitter), so I started to research this issue. Please note, this issue is USA-centric, because it deals with the USA laws, which are different from European or elsewhere when it comes to Cosmetic Ingredients.
- You can see the list of approved ingredients in cosmetics here, the Color Additives Approved for Use in Cosmetics.
- You can see the regulations surrounding ingredients on the Color Additives and Cosmetics page.
- You can check out specific Cosmetic Ingredients in the Skin Deep Database, which tells you about individual ingredients.
- You may remember me linking to these lists a few years ago with my Glowing in the Dark post.
I ended up mailing the FDA, thinking that would clear things up. What transpired was nothing more than a confusing series of emails that left me with more questions.
I emailed:
Is it safe to buy products with glitter in them from MAC or other large cosmetics companies? Is it safe to use nail polish that contains glitter? Is it safe to use eye shadow with glitter? What about eye liner with glitter? Blush or lipstick with glitter? So many of the products I own have glitter in them and I have not had an issue, but I would like to know more.
I did read your page:
Color Additives in Specific Products
but I did not find the information there to be clear enough.
The response I received:
You have asked about the use of glitter in cosmetics. Here is some information that you may find helpful:
Color additives used to achieve variable effects, such as those found in pearlescent cosmetic products, are subject to the same regulations as all other color additives. Glitter usually consists of aluminum, an approved color additive, bonded to an etched plastic film composed of polyethylene terephthalate. FDA considers glitter and mica-based composite pigments to be non-permitted color additives when used in FDA-regulated products, including cosmetics. However, we are exercising enforcement discretion for a period of time. During this time, we will allow glitter and mica-based composite pigments to be released with comment when presented for importation into the US. Once the enforcement discretion period is over, FDA will resume our enforcement of these non-permitted colors.
A list of color additives permitted for use in cosmetics is available on FDA’s website at Color Additives Permitted for Use in Cosmetics.
FDA evaluates the safety of color additives as part of the color additive petition process. So far we have not received a petition for listing glitter in our regulations. To learn about the petition process for color additive approval, please go to ColorAdditivePetitions. Petitions are usually submitted to FDA by color additive manufacturers or trade associations. We encourage petitioners to meet with FDA staff early in the petition process in order to discuss whether additional safety studies will be needed and to obtain other helpful information.
I emailed again, asking for further clarification. I listed specific examples and product ingredients.
The response I received was:
Under U.S. law, cosmetics are not subject to premarket approval by FDA, with the exception of color additives. Cosmetics must not be adulterated or misbranded; that is, they must be safe for consumers under labeled or customary conditions of use, and they must be properly labeled. With the exception of color additives and several ingredients that are prohibited or restricted by regulation (see Restricted/Prohibited, cosmetic firms may use any ingredient, provided it is safe and does not cause the product to be adulterated or misbranded in any other way. Companies and individuals who manufacture or market cosmetics have a legal responsibility for the safety and labeling of their products. FDA can and does take action against products that do not comply with the law, and against firms and individuals who market them.
A cosmetic that is harmful under labeled or customary conditions of use would be considered adulterated—and thus prohibited in interstate commerce—under the Federal Food, Drug, and Cosmetic Act, whether or not it contains a prohibited or restricted ingredient. FDA’s general approach to investigations of the safety of cosmetics includes consideration of such factors as routes of exposure, vulnerable populations, and amounts expected to be absorbed or ingested under conditions of use.
The regulatory status and safety of glitter, mica-based pearlescent pigments, and other composite pigments used in cosmetics are currently under discussion while we are exercising enforcement discretion for these color additives.
So I emailed again, asking for more clarification. The response I received was:
We review this (products) on a case-by-case basis when products are presented for importation into the US. We cannot comment on the safety and/or regulatory status of particular products/ingredients to a third party due to protections granted under the Freedom of Information and Privacy Acts.
Enforcement discretion means that we will allow products into the US that contain certain non-permitted color additives, like glitter, while we continue to review data submitted through the petition process and gathered by other means. Once we have completed our review and made a determination, the enforcement discretion period will end.
I was shocked that they ‘couldn’t comment due to the Freedom of Information and Privacy Acts.’
What I took away from my emails (and this is from reading between the lines and the ‘case-by-case basis’ comment) is that larger companies, such as MAC, will not have these rules surrounding glitter enforced. Smaller companies may be subject to enforcement of these laws.
So what sort of ingredients are safe for glitter?
Calcium aluminum borosilicate, mica, synthetic mica, or cosmetic safe glass-based borosilicates. Companies still have options for adding glitter and shimmer effects to their products while following the guidelines laid out by the FDA
Ingredients to look for that are not approved for cosmetics: (i.e. be wary)
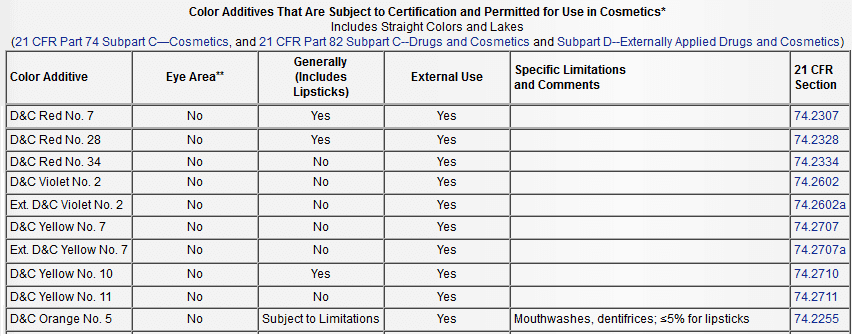
- Yellow 7, CI 45350:1, CAS 2321-07-5 (External cosmetic uses, not Lip and not Eye.)
- Yellow 10, CI 47005, CAS 8004-92-0; 38615-46-2; 94891-32-4
(General cosmetic uses, not Eye.) - Yellow 11, CI 47000, CAS 8003-22-3 (Cosmetic uses, not Lip and not Eye.)
- Violet 2, CI 60725, CAS 81-48-1 (External and rinse off, not Lip, not Eye. )
- *Orange 5, CI 45370:1, CAS 596-03-2 (approved for up to 5% of a lip formula.)
- *Red 7 (not approved for the eyes, approved for blush and lipstick),
- *Red 28, CI 45410, CAS 18472-87-2 (not approved for the eyes, approved for lipstick)
- *Red 34 (not approved for lips)
* Ingredients approved in limited capacity
As such, if I were an independent company owner I would discontinue using any of the ingredients listed above, except for their intended use, such as Red 7 being perfectly safe for blush and lips. It would not be worth it to me to make products from those ingredients and run the risk of being held liable. However, I would also submit a petition to the FDA and get as many people as possible to petition about the glitter additives to enact a change in the law. The only way to enact a change is to have enough people contact the FDA over it.
If you come across a company selling products that list these ingredients that may not approved, you can always ask them about their certification. It should be called a batch certification. If the owner doesn’t have that certification, then the ingredient is likely not FDA approved and may not be safe to use around the eyes.
Additionally, even if a company says ‘this isn’t FDA approved for use around the eyes yet, but it’s approved in other countries’ I would be very cautious with the product. There could still be legal ramifications from the FDA for that company. Or you could end up with permanent damage to your eyes! Many, many people ended up with permanent scarring and damage from Glittersniffer products.
To boil this down a bit:
- There is a lot of vagueness on the part of the FDA with glitter. There are certain ingredients that are definitely not safe and you should avoid them.
- The FDA has not called cosmetic-grade PET microfine glitter, a staple in cosmetics manufacturing, unsafe.
- If you have a reaction to an ingredient, immediately seek medical attention. You do not want to mess around with eye injuries. You can also contact the FDA.
- Always be sure to read the ingredients listing for a product. If it doesn’t have a listing, be very wary.
- You can always check the Color Additives Permitted for Use in Cosmetics list.
This post has been in the making since May. I’ve been thinking and researching on this issue for 2 months and I wanted to share what I’ve learned. I welcome discussion on this hot topic.
Edit: I added a chart from the Color Additives Permitted for Use in Cosmetics FDA page for easy reference for the ingredients I listed above.
This post is accurate to the best of my knowledge. Please contact me if you feel there is more information that should be added or if anything is inaccurate.

I know this is an old article but in lieu of recent news regarding a request for the ban of micro-glitter due to the effects on the environment and ocean life, what is your stance on this issue?
The only glitter I put on my face / eyes is stuff like Urban Decay Heavy Metal Glitter eyeliner or UD Moondusts, or indie brands that use mica that looks like glitter. For nails, I’m less worried about it and am fine with whatever is in my nail polish. I personally think that everyone switching to mica that looks like glitter is a better option for the environment and safer for everyone involved.
Since I’ve had allergic reactions to a specific type of mica, and problems with some products, I’ve been nervous about the size of glitter.
When I was 7, I scratched up my corneas while playing with my mom’s makeup. It was very scary for a little kid, but I doubt I’d be very thrilled to have the same thing happens now and have to deal with doctors, meds, and insurance.
The way I see it, the FDA is kind of doing like drug companies do when they list possible side effects, they’re just taking it one step further. Considering how young some girls are when they start wearing makeup, I’m kind of glad the FDA wants to keep ingredients that are known to be dangerous out of cosmetics, because while a lot of parents are careful about what medications their kids take, how many of them pay much attention to the makeup their daughters wear, besides whether they’re wearing too much or too young to wear it at all.
My opinion I would not use glitter near your eyes. I am always afraid the glitter would get in my eyes and cut it. I have seen certain companies that sell glitter with their sealer for eyeliner & shadows at trade shows and TMS Chicago scares the beegeebies out of me
You wouldn’t BELIEVE the number of people who don’t go see their doctor when they have a reaction! Crazy!
My reason for writing the post is that:
There is a lot of vagueness on the part of the FDA with glitter. There are certain ingredients that are definitely not safe and you should avoid them.
If you have a reaction to an ingredient, immediately seek medical attention. You can also contact the FDA.
Always be sure to read the ingredients listing for a product. If it doesn’t have a listing, be very wary.
I want to prevent another Glittersniffer, where people have permanent eye damage.
what I don’t understand is why anyone gives half a fig what the FDA thinks. if I have a problem w/a cosmetic, I’ll stop using it, but it’s up to ME to make that determination. I just want the State to LMTFA.
@CreativelyYours You’re welcome 🙂
Great article, Phyrra! Seems like a huge bureaucracy, to me. :/
@KimberlyisHere It really is!
Great post! It’s always best for people to know what’s in their makeup before using it.
@lolbeauty Thank you! The only disappointing thing to me is that it’s not as straight forward as I’d like (as a consumer).
This is great info to know Phyrra, thanks for sharing!
@FrancesDanger It was very strange. That’s one of the reasons I mentioned the petition, because the only way to enact change is to get enough people to ask them.
@Phyrra Ok, I misread that. FDA states no one has petitioned thus far to get glitter approved. That makes me wonder what they can’t disclose under FOIA. If there’s no petition there’s no third party info to protect.
@stefanily I agree with you that the ambiguity is hurting us as consumers and small businesses 🙁
@Phil Grier Thank you! And you raise a really good point. If businesses have no motive to invest, things won’t change or move forward.
@Phil Grier @stefanily I worry because I don’t think enough people know that they can even contact the FDA.
@stefanily I do agree that the ambiguity is hurting small business 🙁
@Hottie I know! I wish it were less complicated.
@FrancesDanger @Sparklecrack Central @Sirvinya Thank you so much for the feedback! Yeah when I specifically asked the FDA about glitter products that I’ve used safely with no issues, they wouldn’t comment on them.
@gothchiq I worry about the idea of getting glitter under my contacts, in my tear ducts, etc.
@Phil Grier That’s a really good point!
@Morganas_Crypt Thank you for sharing this!
@Phyrra @Sparklecrack Central @Sirvinya It is my understanding that the reason that *some* ingredients are approved in the EU and not in the states is simply because they have not yet been petitioned for addition or tested to be approved for use as per FDA.This only applies to some, not all, ingredients that are not approved. “Not approved” does not automatically mean unsafe. That being said I don’t advocate smearing non FDA approved substances on your eyeballs.
Take, for instance, the Neons. The soap dyes as sold by TKB are not approved for cosmetic use. A quick glance at the MSDS shows that the uses are too numerous to be included in 21 CFR and that each potential use would have to be submitted individually for inclusion. That means separate petitions and testing for each use. Too costly. Better to just leave it as a soap dye as the testing for the color additives is not undertaken by FDA, but the petitioner, who bears the cost. We don’t really know if the neon soap dyes are unsafe, just unapproved (but I would not go putting them on your lids either as the MSDS also shows they can cause irritation and just because it hasn’t yet been tested doesn’t mean that when it is it will be approved for eye use).
So why is something like the Sleek Acid Palette approved in the EU and not the states? For one thing, it contains Red 27, which is not approved for eye use in the US. As to why there is a disparity between the approvals, well, you got me. I do know that before any cosmetic goes to market in the UK it must be chemist certified. FDA does not test or approve finished cosmetics prior to their launch into interstate commerce. They simply regulate what’s approved for use in the cosmetic.
In terms of the glitter debate it seems pretty silly to me that an ingredient that has been in use for years with very little adverse reaction would now be under scrutiny. But as it imparts color due to the pigment inside the plastic it does meet the definition of a color additive and as such would need FDA approval for use. I would like to see it approved so its use is above board. I also believe there needs to be a regulation on the size of glitter that can be safely used as well. The thing is, since this falls on the petitioner, it is going to take a major cosmetic manufacturer or a glitter manufacturer such as Meadowbrook to not only submit the petition, but gather and pay for all the information and testing supporting its approval. FDA doesn’t test for approvals. It simply reviews the petitioners data and makes a ruling based on that information. It sounds as if there have already been petitions submitted based on the emails from FDA so I guess we wait and see.
This was an excellent post, Phyrra. I know from experience that getting an answer from FDA is like wading through molasses: slow going and pretty annoying. Hopefully someone has started the petition process and the matter can be put to rest soon.
Fudgesicles, my eyes started to glaze over reading those responses, hahaha! The protections they’re talking about under FOIA probably refer to information that is statutorily exempt from FOIA disclosure–we’re talking things like internal management and confidential information belonging to non-agency parties, including information in the nature of privacy. So for example, if what they’re alluding to here is trade secret protection (which sounds the most likely if they’re unable to comment on individual formulations), the one legal protection trade secret in particular has is the fact that the protected thing is actually a secret (which is why it’s generally thought of as the weakest form of IP protection available in the United States). If the agency can’t comment on something without disclosing confidential information that is the property of another, it isn’t public information–so it shouldn’t be susceptible to FOIA to begin with.
And also, thanks for all of the research you did on this post! Great job!
@Ciambella Yeah I didn’t think it should fall under FOIA. Their responses were so convoluted.
Hi! I liked your blog, it looks interesting! I offer you my sincere friendship!=)Please, follow me on Bloglovin, if you will like my blog: http://www.bloglovin.com/en/blog/3831007/ko-tecom-kisses-from-europe
I will follow you via Bloglovin;) Let’s support each other! ^^Love&kiss!
Hi! I liked your blog, it looks interesting! I offer you my sincere friendship!=)Please, follow me on Bloglovin, if you will like my blog: <a href=”http://www.bloglovin.com/en/blog/3831007/ko-tecom-kisses-from-europe”>Kisses From Europe</a> I will follow you via Bloglovin;) Let’s support each other! ^^Love&kiss!
Great post Phyrra! I find the whole thing a bit baffling. I’m learning to make cosmetics myself and lots of ingredients which are not approved for use by the FDA are considered fine over here. For instance the EU considers it safe to use chromium oxide green in lip products while in America that’s not allowed. So… In a way it makes it worse! I’m not going to avoid ingredients that are considered safe here but not in the US, but then what if the FDA is right? I think the glitter thing is a bit stupid though as the colorant is trapped in the plastic or whatever.
@LillianFunnyFace I think what some companies do is only sell some products to the USA because of the colorant issue.